Extra sense for surgery
ICON3D®
ICON3D® is a SaMD (Software as a Medical Device) for the intra-operative visualization and manipulation of HA3D® virtual anatomical models.
The purpose of ICON3D® is to provide support for complex surgeries, improving the user experience of the surgeons and allowing them to reproduce the pre-operative planning during surgery.
On the monitor of ICON3D®, the doctors can independently and interactively review the resections, annotations and relevant details that they have studied, following the planned sequence of surgical steps.
Cognitive Surgery
The intended use is as an Auxiliary Display. The surgeons can look at the ICON3D® monitor and increase their perception of the real patient’s anatomy, to better recognize the anatomical structures and reproduce the pre-operative planning, previously studied on MyMedics portal.
Augmented Reality
ICON3D® allows surgeons to view endoscopic video streams as 3D reconstruction background, providing an Augmented Reality solution for rigid and semi-rigid anatomical structures.
Touchless interaction
ICON3D® can be used in combination with standard mouse and keyboard, a hand tracking sensor or the innovative Medics product M3DICON, composed of a 3D mouse and a keypad. In this way, ICON3D® allows surgeons to easily visualize and manipulate HA3D® models. Through a dedicated smart menu, the surgeon can access all the functionalities of the software. In open and laparoscopic surgery, the HA3D® reconstructions can be visualized with a specific device, that ensembles the flexibility of a tablet and the performance of a PC. In robotics ICON3D® is displayed with the split-screen function within the robotic consoles.
Smart Versatile Interaction
ICON3D® enable collaboration within the operating room, making HA3D reconstruction avaiable for the entire surgical team.
In the meantime, the other members of the team can always input commands by standard mouse and keyboard, enforcing team cooperation in any condition.
What kind of Surgeries?
In open and laparoscopic surgery, the HA3D® reconstructions can be visualized with a specific device, that ensembles the flexibility of a tablet and the performance of a PC.
In robotics ICON3D® is displayed with the split-screen function within the robotic consoles.
ICON3D® is a plug and play solution, already set up and ready for use in all surgeries
Why you should use ICON3D®
- The use of ICON3D® reduces the risk of intra- and post-operative complications.
- Intraoperative use of ICON3D® returns patients who benefit from more conservative surgeries.
- ICON3D® accurately represents patient anatomy and pathology in easy-to use anatomical models, providing intraoperative support for in vivo identification of anatomical structures.
- The versatility of ICON3D® makes it usable by any surgeon and for any type of surgery.
The ICON3D-S System
Medics provides ICON3D-S, a system with ICON3D® software and compatible hardware for use in the operating room.
ICON3D® as a SaMD (Software as Medical Device) can be used in combination with dedicated input devices and third-party sources.

M3DICON
M3DICON is an input devices composed of a 3D mouse and a keypad with descriptive icons to allow an easy and intuitive interaction.
DEDICATED DEVICE
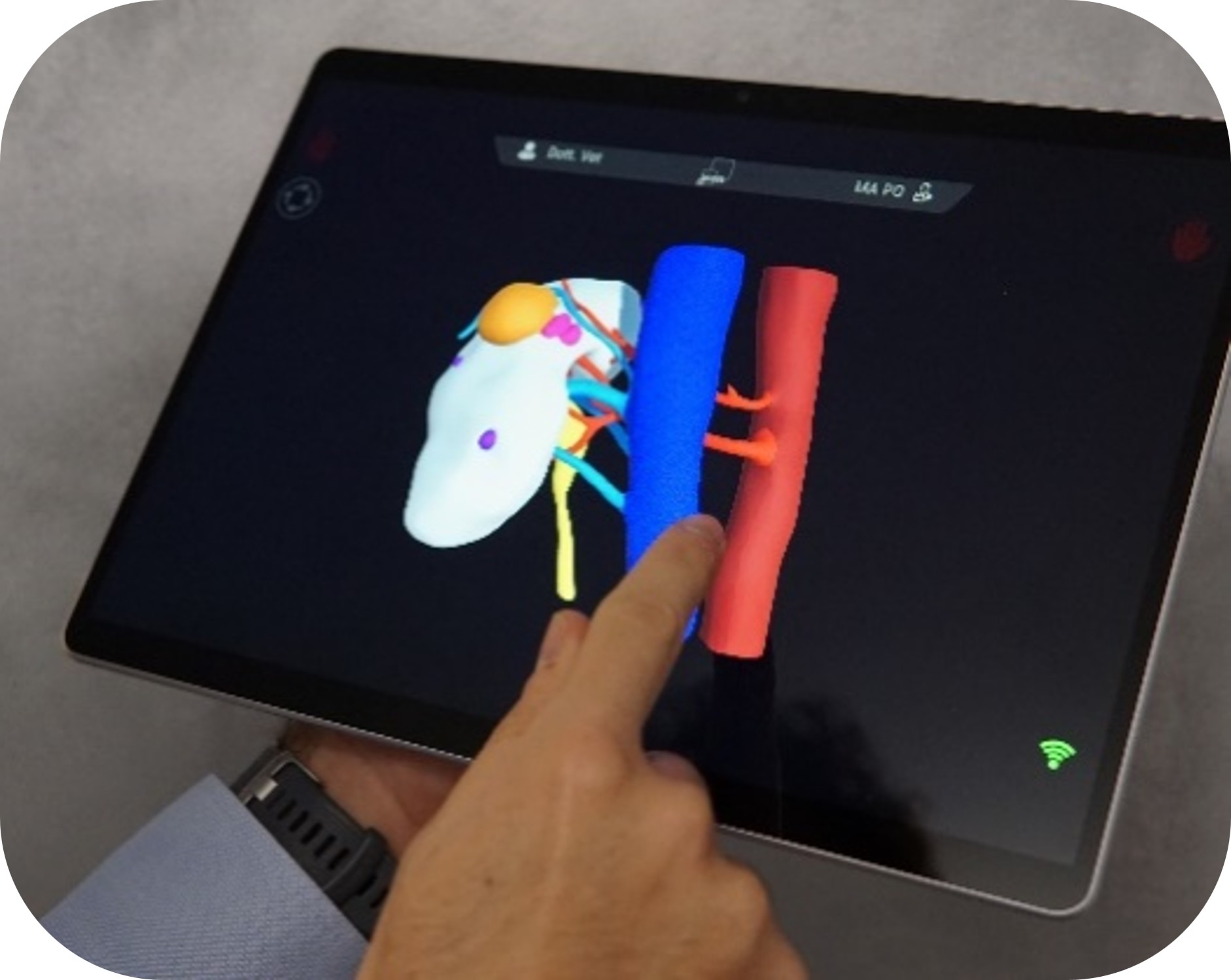
In the system ICON3D-S LIGHT, the software ICON3D® is installed on dedicate device, that ensembles the flexibility of a tablet and the performance of a PC.
The anatomical models displayed on ICON3D® are virtual HA3D® reconstructions previously realized, uploaded and studied on MyMedics cloud portal.
ICON3D® makes available all the annotations and the surgical planning previously elaborated by means of HA3D®, collecting them within customizable preset views conceived to be recalled during the surgery. To view the three-dimensional reconstructions on ICON3D® ‘s display, just log-in in the software using your MyMedics credential and download the HA3D® models directly from the Operating Room (it is necessary to have an Internet connection inside the OR).
ICON3D® is a Class IIa Medical Device with CE marking, in compliance with the Medical Device Regulation (EU) 2017/745.
